Life History & Behaviour
Reproduction
Pyrazus ebeninus experience gonochoristic reproduction meaning these organisms have only one sex throughout their life and do not experience hermaphrodism. Reproduction through a deep, ciliated egg groove present on right side of foot in females that produces gelatinous egg strings. (Strong et al. 2011). The presence of an egg grove and gonochoristic reproduction implies that reproduction occur through external fertilization with no anterior copulatory bursa present in females and genital ducts originating on the right gonoduct and renal duct (Ruppert et al. 2004). The ovipositor is located on an external pad and the pallial oviduct in these organisms is almost or completely open similar to ancestral gastropods. Unlike some other Potamodids, P. ebeninus has two sperm gutters that are located in the lateral pallial oviduct medial lamina compared to either one or none seen in other gastropods (Houbrick, 1991). The spematophore bursais present in medial lamina, but is absent in the lateral side. The seminal receptacle in medial lamina is derived from the pallial oviduct and is contained within the laminae. There is a cephalic penis in this genus located on the muscular foot head behind the cephalic tentacles. The male pallial gonoduct is closed and there is no spermatophore forming organ in prostate, though spermatophores are present in the body (Strong et al. 2011). The gonad is positioned mainly dorsal to digestive gland. These organisms have free swimming veliger larva stage, but the chromosome number is unknown (Edwards 2009).
Gametes
The mature acrosomal vesicle shape is conical and basally flattened on the anterior side of the rod forming a basal invagination (Healy, 1982). The acrosomal vesicle apical bleb is present with the acrosomal vesicle basal invagination sized at half or less of the full length of the vesivle (Strong et al. 2011). The mature nucleus shape is straight and densely backed with electrons (2.9 mm), while the nuclear basal invagination length is short (0.9 mm) (Healy 1982, Fig. 2). The mature midpiece is surrounded by 4 mitochondrial elements of two small block shaped pieces and twolarge semi-circular like pieces, which suggests helical arrangement (Healy 1982). The mitochondrial cristae are unmodified and irregular with a simple mitochondrial neck. The sheath is absent and two paraspermatosoal are present. The acrosomal structure is exhibited by a membrane bound vessel filled of dense material, known as the basal invagination (Strong et al. 2011). The axonemal number is known to be about 2-3. The emergent asposterial tail tuft with condensed, elongated nucleus present with central rod (Fig. 2; Healy 1982). The axoneme is attached to nucleus only and penentration of the head occurs to the base only (Healy 1982). Large dense, homogeneous vesicles and a dense ring structure are also seen around the base of the vessel (Fig. 2).
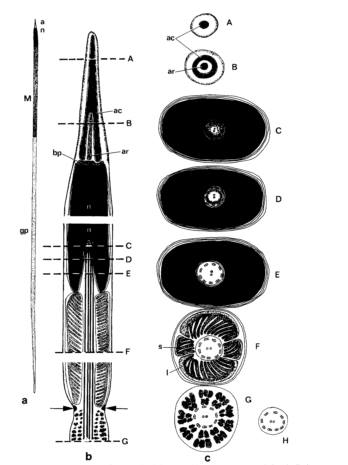 
Figure 2: P. ebeninus euspermatozoon diagram with a) euspermatozoon b) longitudinal and c) transverse section of euspermatozoon-acrosome (levels A, B), nuclear invagination (levels C, D, and E), mid-piece (level F), glycogen piece (level G), and end piece (level H). The dense ring structure is indicated with arrows. (Figure taken from Healy 1982)
Movement
P. ebeninus moves with a large muscular foot that protrudes from the flared opening at the bottom of the shell (Fig. 3). The muscular foot size ranges varying on shell length. The hypobrancial gland, found in the epithelium of the pallial cavity, is well developed and produces mucous used for the muscular foot movement (Pontarotti, 2010). The strength of the muscular foot is tested in the Anatomy & Physiology section of this website against varying substrate environments. (See "Muscular Foot Strength" background section)

Figure 3: P. ebeninus specimen seen motile in the sample tray with fully extended foot and cephalic tentacles displayed at the anterior end of the specimen.
Respiration & Internal Transport
Similar to other land snails, these organisms can stay out of water for long periods of time because they have a fleshy, pallial cavity that contains gills similar to a lung that are used for respiration (Edwards 2009). Due to that gastropods are small compact organisms, they have a hemal transport system without a coelom present. The system of gastropods is similar to other molluscs with the exception that the paracardial cavity and heart are located in the anterior visceral mass as a result of torsion (Ruppert et al. 2004). The blood transported by gastropods is primarily used for internal transport, but also serves as a hydrostatic skeleton. The hemocoel is likely to be a large sinus in areas where blood is hydraulic, whereas in regions where blood is mainly the exchange of materials with tissues, the hemocoel is likely to be capilary like (Ruppert et al. 2004). Due to that P. ebeninus are not freshwater organisms, they a respiratory pigment called hemocyanin in their plasma. The ventricle in gastropods typically receives oxygenated blood through the process of blood that is transported to through the aortae that supplies blood the visceral hemocoel and anterior region that is ultimately fed into the cephalopedal hemocoel (Ruppert et al. 2004). All remaining unoxygenated blood is returned to the heart through the afferant branchial vessels of the gills to become oxygenated and returned through the efferant branchial vessels to the atrium.
|